Radioligand therapy for advanced prostate cancer patients – are health care systems prepared?
date: 21/02/2024
This targeted, systemic molecular radiotherapy can improve survival and quality of life of patients with disseminated, metastatic cancer. However, the high number of potentially eligible patients can lead to a shift of cancer treatment towards nuclear medicine requiring treatment capacity for which not all European health care systems may be prepared (e.g. in terms of equipment and specialised workforce). It is important to assess such capacities as well as reimbursement decisions if we are to to leverage on the potential of these therapies and integrate them into clinical practice.

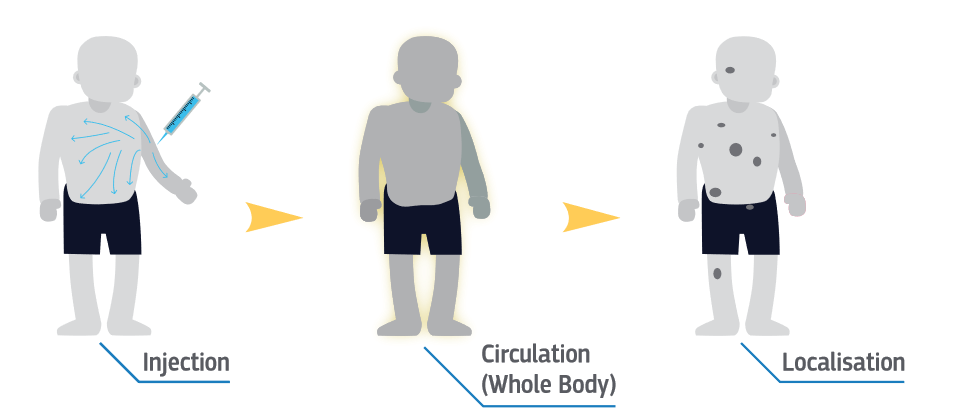
Figure: Scheme of a targeted systemic molecular radiotherapy. The radiopharmaceutical is injected intravenously and circulates in the body until it finds its target cancer cells and irradiates the lesions.
