Ethics
For all activities funded by the European Union, ethics is an integral part of research from beginning to end, and ethical compliance is seen as pivotal to achieve real research excellence. There is clear need to make a thorough ethical evaluation from the conceptual stage of the proposal not only to respect the legal framework but also to enhance the quality of the research. Ethical research conduct implies the application of fundamental ethical principles and legislation to scientific research in all possible domains of research. The process to assess and address the ethical dimension of activities funded under Horizon 2020 is called the Ethics Appraisal Procedure.
Objectives
In addition to the scientific evaluation focusing on the scientific merit, the quality of the management and the potential impact, the Ethics Appraisal ensures that all research activities carried out under the Horizon 2020 Framework Programme are conducted in compliance with fundamental ethical principles.
Ethics Appraisal Procedure
The Ethics Appraisal Procedure concerns all activities funded in Horizon 2020 and includes the Ethics Review Procedure, conducted before the start of the project, as well as the Ethics Checks and Audits.
When preparing a proposal, it is required to conduct an Ethics Self-assessment starting with the completion of an Ethics Issues Table. You can read further practicalities in How to complete your ethics self-assessment guide.
Ethics Review Procedure
All proposals above threshold and considered for funding will undergo an Ethics Review carried out by independent ethics experts and/or qualified staff working in a panel. The Review starts with an Ethics Screening and if appropriate a further analysis called the Ethics Assessment is conducted. The Ethics Review can lead to ethics requirements that become contractual obligations.
The Ethics Review Procedure focusses on the compliance with ethical rules and standards, relevant European legislation, international conventions and declarations, national authorizations and ethics approvals, proportionality of the research methods and the applicants' awareness of the ethical aspects and social impact of their planned research.
- Ethics Screening
-
The first phase of the Ethics Review Procedure, the Ethics Screening, is carried out during the scientific evaluation or soon after. The ethics experts and/or qualified staff first perform an Ethics Pre-Screening taking into account the Self-assessment. The objective of the pre-screening is to list the (potential) ethical issues but not to assess them.
When there is at least one confirmed ethical issues, the proposal is subject to a complete Ethics Screening that will mainly assess the ethical aspects of its objectives, methodology and potential impact. The ethics experts and/or qualified staff will notably identify all proposals that require (ethical) approval at the national level (e.g. with regards to data protection, the conduct of clinical trials and animal welfare). Because of the complexity or the nature of the ethical issues they at stake (e.g. severe intervention on humans) the ethics experts and/or qualified staff may also recommend an Ethics Assessment rather than formulating directly requirements. Proposals involving the use of Human Embryonic Stems Cells (hESCs) automatically proceed to the second step, the Ethics Assessment.
- Ethics Assessment
-
For a limited number of proposals (e.g. severe intervention on humans, lack of appropriate ethics framework in the country where the research will be performed, etc.) the Ethics Screening can be followed by an Ethics Assessment prior to the signature of the grant agreement.
The Ethics Assessment is an in-depth analysis of the ethical issues of the proposals, taking into account, when available the conclusions of the Ethics screening. As mentioned above, it is systematically performed on all proposals involving the use of Human Embryonic Stem Cells.
Ethics requirements and Ethics work package
There are two types of ethics requirements. Requirements that you need to comply with
- during grant preparation
- during the ongoing project
Ethics deliverables: All ethics requirements due after project start are automatically included in the grant agreement in the form of deliverables. These deliverables are known as 'ethics deliverables' and will be placed in an automatically generated work package called 'ethics requirements'.
Read more grant preparation related ethics information.
Ethics Checks and Audits
During the Ethics Screening or the Ethics Assessment, the experts identify the projects that need an Ethics Check, which are executed during the course of the research project. The procedure can also be initiated by the Commission services.
The objective of the procedure is to assist the beneficiaries to deal with the ethics issues raised by their research and if necessary to take preventive or/and corrective measures. The Ethics Check is conducted on the basis of the information provided by the concerned beneficiaries, who may be invited to a meeting in Brussels to discuss the issues at stake. On site visits can also be organised.
In case of substantial breach of ethical principles, research integrity or relevant legislation, the Commission can carry out an Ethics Audit following the provisions and procedures laid down in the grant agreement.
The Checks and Audits can result in an amendment of the grant agreement. In severe cases, it can lead, upon the decision of the Commission services to a reduction of the grant, its termination or any other appropriate measures, in accordance with the provisions of the grant agreement.
ETHICS APPRAISAL STEPS
| Activity | Who? | When? | How? |
|---|---|---|---|
| Ethics Self-assessment | Applicant | Application phase | Consideration of ethical issues of the proposal |
| Ethics Pre-screening/Screening |
Ethics experts and/or qualified staff | Evaluation phase | Review of application material |
| Ethics Assessment (for proposals involving hESC or raising serious ethical issues: severe intervention on humans) |
Ethics experts | Evaluation/ Grant preparation phase |
Review of application material |
| Ethics Check/Audit | Ethics experts | Implementation phase | Review of project deliverables/interview with applicants |
The Ethics Appraisal Procedure is established by the Rules for submission of proposals, and the related evaluation, selection and award procedures.
More general information and an e-Library providing access to the most important pieces of European legislation, international conventions and declarations and codes of conduct relevant to research activities can be found on the Europa website.
For assistance please contact us at the Ethics Review Helpdesk (select subject 13. Ethics in the enquiry form).
Utilisation of Genetic Resources: Obligations under the EU Regulation on Access and Benefit Sharing (ABS)
As a recipient of EU research funding you have certain obligations under the EU Regulation on Access and Benefit Sharing (ABS) if you utilise genetic resources. In particular, you must
- determine if your project falls within the scope of this regulation, and
- if yes, ensure your project is compliant
This section helps you decide if your project is in scope and provides information on the obligations that have to be met. An introduction to Access and Benefit Sharing (ABS), an overview of the ABS legislation, and useful links are available on the Europa website.
For IT help on completing the ABS regulation tab, please see here: 
Check if your project is in scope of the ABS regulation
A project is in scope if it meets all of a number of conditions (cumulative conditions). In order to establish if your project is in scope you can apply the decision tree below. It contains all relevant conditions. Depending on your answers the decision tree will return 'out of scope' or 'in scope'.
It is highly recommended that you also consult the guidance document on the scope of the EU ABS Regulation. This guidance was published by the European Commission to clarify the provisions of the EU ABS regulation. It explains all conditions that define the scope of the regulation (including concrete examples from specific sectors) as well as the obligations that arise if your project is in scope.
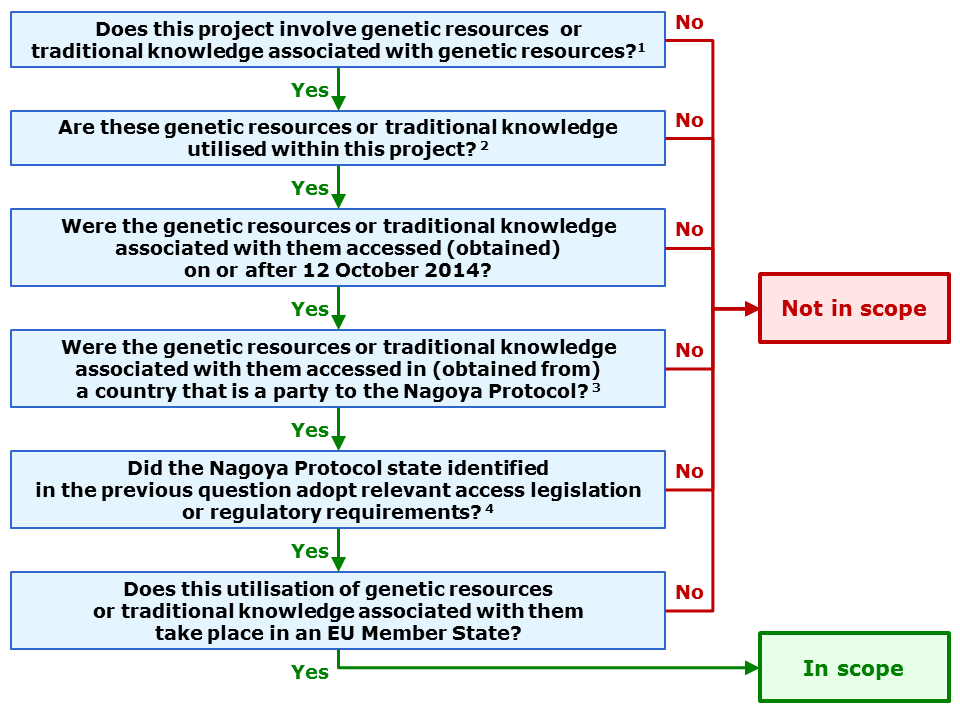
In scope: This project falls within the scope of the EU ABS Regulation. Please report this result through the Funding & Tenders Portal Grant Management Service's continuous reporting module (see Obligations for projects in scope of the ABS regulation section below).
Not in scope: This project does not fall within the scope of the EU ABS Regulation. No further action is needed unless the reply changes during the lifetime of the project. The non-EU participants utilising genetic resources or traditional knowledge associated with them within this project must comply with the Nagoya Protocol regulations in force in the country where they are established.
1 For a definition of "genetic resources or traditional knowledge" within the meaning of the EU ABS regulation, see the ABS scope guidance document.
2 For a definition of "utilisation" within the meaning of the EU ABS regulation, see the ABS scope guidance document.
3 See full list of Nagoya Protocol states. Please note this list includes some EU countries (i.e. if the material or knowledge originated from one of these EU countries you must reply 'yes').
4 The applicable laws (i.e. the legislative, administrative or policy measures, if any) in each Nagoya Protocol state can be found here. To find out if the access to genetic resources is regulated in the country concerned, you also need to check with the 'focal point' of this country. Contact information for the ABS national focal points is available on the same webpage. If no access legislation or regulatory requirements are in force in the country concerned, access is considered to be free.
Obligations for projects in scope of the ABS regulation
If your project falls within the scope of the ABS regulation you must
- report that your project is in scope before you receive the first payment (the pre-financing is not considered a payment for this purpose)
- Locate the 'My Area' section of the Funding & Tenders Portal Grant Management Service.
- Go to the 'My Projects' list.
- Click on the 'Actions' button, then on 'Manage Project'.
- Select 'ABS regulation' in the 'Continuous reporting' module.
- comply with the ABS Regulation, in particular
- exercise due diligence
- submit a due diligence declaration
Exercising due diligence is the core obligation under the ABS regulation. In addition to the regulation itself, you should consult the guidance document on the scope of the ABS Regulation to understand the relevant obligations and ensure compliance.
Due diligence declaration
The due diligence declaration must be submitted to the competent authority of the member state where the coordinator or beneficiary is established. The contact details of these competent authorities are available on the Europa website. As soon as possible the Commission will provide an online submission tool that will direct the declaration to the appropriate authority.
- For multi-beneficiary grants, the project coordinator may make a single declaration. Alternatively, each beneficiary whose activities fall within the scope the EU ABS Regulation must make an individual declaration.
- The declaration must be made at the latest by the end of the project (final report).
- If the coordinator or beneficiary is established outside the EU and the relevant research activity takes place inside the EU, the declaration must be submitted to the competent authority of the Member State where the research is carried out.
- Beneficiaries established outside the EU and carrying out research outside the EU must comply with their own national ABS legislation (if any). They are not concerned by the ABS regulation of the EU.
Rules & codes of conduct
- Legal basis - Horizon 2020 Rules for Participation: Ethics Reviews (Article 14)
- Horizon 2020 - Regulation of Establishment: Ethical principles (Article 19)
- Model Grant Agreement: Ethics (Article 34)
- Statements by the Commission on human embryonic stem cell research
- Guide for proposal submission and evaluation
- Charter of Fundamental Rights of the European Union
- European Code of Conduct for Research Integrity
- Global code of conduct for research in resource-poor settings
General guidance
Domain-specific guidance
- Guidance note — Research involving dual use items
- Guidance note — Potential misuse of research results
- Guidance note — Research focusing exclusively on civil applications
- Guidance note — Research on refugees, asylum seekers & migrants
- Ethics and data protection
- Ethics in "Science with and for society"
- Ethics in Social Science and Humanities
- Position of the European Network of Research Ethics Committees (EUREC) on the Responsibility of Research Ethics Committees during the COVID-19 Pandemic

