by Dr. Martina Pötschke-Langer MD, M.A., Head of Unit Cancer Prevention WHO Collaborating Centre for Tobacco Control, German Cancer Research Center (DKFZ)

On May 20th, the new Tobacco Products Directive (TPD) became applicable in the EU Member States. The Directive is an important achievement for public health in the EU and aims in particular to discourage young people from smoking.
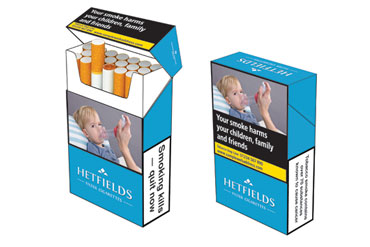
The Directive will bring major changes to the tobacco products sold in the EU. Flavoured cigarettes that are attractive to young people such as fruit or menthol will not be allowed. Cigarette packages will carry large combined health warnings that remind consumers of the risks of smoking. Tobacco companies will not be able to market their products through misleading terms such as ‘organic’ or ‘natural’. The Directive also addresses the illicit trade of tobacco products. New provisions for electronic cigarettes will ensure that these products are no longer unregulated in the EU and meet specific safety and quality requirements.
The past two years have been an intense time for those of us working in tobacco control.
The European Commission has adopted nine legal acts (eight implementing acts and one delegated act) containing the detailed technical rules needed to implement the TPD. These include the methods to determine flavours in tobacco products, the appearance of the new health warnings and rules for refillable electronic cigarettes.
The European Parliament and the Council have defended the Directive in three separate cases brought by the industry and one Member State. On May 4th, the Court of Justice declared the Directive valid on all points, confirming its internal market basis and ensuring that its public health benefits are not lost.
Member States have also worked hard to transpose the Directive. Many have introduced complementary or further measures in their Tobacco Acts such as plain packaging, advertising restrictions or smoke-free environments legislation.
For those of us working in the tobacco control community, the fight for a health oriented and evidence based TPD was a huge task – especially when faced with massive lobbying by the tobacco industry and its allies. This difficult task also continued in the Member States as we supported them to transpose the Directive.
The application of the TPD across the EU is a big step forward to ensure the health of all our citizens. We have seen that smoking prevalence is falling in the EU. The TPD should reinforce that trend and ensure that fewer young people take up this deadly habit.