Archive:Morbidity statistics methodology pilot studies - introduction
16 Member States conducted pilot studies for testing a methodology on diagnosis-specific morbidity statistics from 2005 to 2011. Subsequently a Eurostat Task Force was established for an in-depth analysis and recommendations in view of the feasibility of such data collection, namely regarding sources and best estimates.
This report summarises the results of the Task Force that was presented to the Working Group on Public Health Statistics In December 2013
Executive summary
Eurostat activities in the domain of health status and health determinants are currently covered by surveys; however, one core activity is missing: the regular collection of diagnosis-specific morbidity data with incidence and prevalence rates. A legal basis for such data collection is provided by Regulation No 1338/2008 establishing a framework for Community statistics on public health and health and safety at work. That regulation foresees an implementation of morbidity statistics at EU level.
Eurostat’s commitment in developing the conceptual and methodological framework for establishing such data collection on morbidity has a long-standing tradition; however, this ambitious goal has not yet been achieved. The reason behind is that from a methodological and operational point of view, establishing morbidity statistics is an extremely complex exercise, in particular with regards to comparability of data across countries. To guide the MS in the piloting phase, detailed guidelines were produced by the Morbidity Statistics Development Group in 2007: for each entry in the recommended shortlist the appropriate measures on incidence and/or prevalence for data delivery were indicated. Each country had to find appropriate sources for producing best national estimates. The main criteria for the inclusion of a data source was statistical robustness on the main data quality parameters and hence permit reliable inter country comparisons. Hence, like many Eurostat statistics, the compilation of diagnosis-specific morbidity statistics is output driven and not source oriented.
Altogether 16 MS participated at pilot studies on diagnosis-specific morbidity statistic from 2005 to 2011. In 2011, Eurostat established the Task Force on Morbidity Statistics (TF MORB) for analysing the pilots’ results, especially in view of sources and best estimates. TF MORB is presenting this report with an indepth analysis of the pilot studies and methodological recommendations for paving the way ahead to overcome the pioneering stage.
The establishment of diagnosis-based morbidity statistics will be crucial for filling an information gap on the health status of the EU population which has severely hampered the development of public health indicators at EU level.
The draft of this report has been presented and discussed at the Technical Group on Morbidity in June 2013; the final version has been presented at the Working Group on Public Health Statistics (WGPH) in December 2013 which endorsed it.
The importance of having European statistics on diagnosesbased morbidity
Eurostat activities in the domain of health status and health determinants are currently covered by three surveys: the five-yearly European health interview survey (EHIS); the newly established disability survey (European Survey on Health and Social Integration - ESHSI), and a basic set of health-related questions that are included in the annual EU Survey on Income, Social Inclusion and Living Conditions (EU-SILC).
However, one core activity is missing: the regular collection of diagnosis-specific morbidity data with incidence and prevalence rates. A legal basis for such data collection is provided by Regulation 1338/2008 establishing a framework for Community statistics on public health and health and safety at work. That regulation foresees an implementation of morbidity statistics at EU level. The Community action Programme on Public Health 2008–13 and the Community Statistical Programme 2008–12 foresee the implementation of that Regulation as a key statistical element of a sustainable health monitoring system. In addition, the Commission Communication Solidarity in health (COM/2009/0567 final) also emphasises the importance of having Regulations developed in each domain of public health statistics.
The recent Commission staff working document Investing in health (complementing the Commission Communication ‘Towards Social Investment for Growth and Cohesion’, available as PDF), which is an accompanying document to the ‘Social Investment Package’, defines the role of health as part of the Europe 2020 policy framework and points out that an improvement in health data collection is needed, in particular in using the European Core Health Indicators (ECHI) and developing tools to better assess the efficiency of health systems. In addition, the statistical information on specific chronic diseases is a key component in underpinning and addressing policies to improve the labour market participation, risk of social exclusion and risk of poverty.
Eurostat’s commitment in developing the conceptual and methodological framework for establishing a data collection on morbidity dates back to the mid-nineties. Following the analysis of pilot studies in 16 Member States this report of the Eurostat Task Force on Morbidity (TF MORB) is now paving the way ahead to overcome the pioneering stage with a set of recommendations.
The feasibility of such statistics, in particular in view of using data from different sources, will be markedly enhanced by the current revision of the EU statistical law. It will be the legal basis to ensure and encourage a better use of existing sources by improving access to and exploitation of administrative data, e.g. by merging or linkages of the existing datasets.
The establishment of diagnosis-based morbidity statistics is crucial for filling an information gap on the health status of the EU population. Key elements of innovation for that approach are:
- the best estimates from multiple sources that can be used (namely physicians issuing diagnoses/prescriptions or health records from registers, health institutions and insurances)
- the possibility to compare best estimates on incidence and prevalence of diseases
- the comprehensive coverage of morbidity data
- the coverage of the whole population, by providing national estimates, and
- diseases and conditions to be reported in terms of EU relevance and the Public Health perspective.
In 2007 Eurostat and Member States (MS) developed a methodology and a shortlist for collecting such data at EU level. It addresses diseases and conditions with major impact on health care and health-care related costs, annual death rates, or potential years of life lost. Examples range from heart/circulatory and respiratory diseases, cancer or metabolic diseases such as diabetes to mental diseases, injuries and their consequences and external causes. An attempt to highlight the main diseases for which morbidity statistics are needed is presented in the EU short list on morbidity, which has been followed throughout this pilot phase by the participating countries.
The actual lack of systematic and official data on morbidity has severely hampered the development of public health indicators at EU level which are required to support health policy makers.
Diagnoses-based morbidity statistics at EU level: a difficult exercise
For selected diseases the health status of the EU population is known thanks to diseases-registers, ad-hoc studies and as self-reported information from the EHIS or EU-SILC surveys. Currently, the principal and most reliable source for establishing and monitoring public health policies is information derived from Causes of Death statistics. While this type of source is well established and provides reliable and comparable public data collection for all EU countries, Cause of Death data does not provide information on incidence and prevalence of diseases and in particular lacks information on comorbidities that would be necessary for a comprehensive picture of public health.
A regular and systematic data collection and dissemination of statistics[1] on diagnoses-based morbidity does not exist either at EU or at global level[2]. The reason behind this is that from a methodological and operational point of view, the collection of morbidity statistics is an extremely complex exercise, in particular with regards to comparability of data across countries. Specific efforts will be required in each country to produce operational definitions of variables that are based on many different available sources. So far these difficulties have hampered attempts to establish a morbidity data collection based on (mainly) administrative data similar to those already existing for causes of death or for health care data based on hospital discharges. The recent new release of the work on the Global burden of diseases (11) is a tentative step towards filling in the existing information gap on health.
The demand for statistical data on diagnosis-based morbidity is increasing; however, the capability to respond to this increasing demand is constrained by limited data availability, quality, and use. A set of diagnoses-based morbidity indicators have been developed in the context of the ECHI (European Core Health Indicators (12)) list, but most of these indicators are not collected yet and the list is not exhaustive. It is therefore important to collect morbidity statistics in order to have these indicators thoroughly implemented both in terms of definition and data. (13)
The paradox for Europe is that while sometimes there is a wealth of information available for specific diseases; this information can often be scattered, sparse, not representative of the total population, not collected systematically and not addressing the multidimensional characteristics of health. And for many other diseases there are only scarce examples at national level. The result is:
- a fragmented picture of the occurrence of diseases in the EU, often driven by the needs of singledisease
program or ad-hoc data collection;
- information on incidence (or prevalence) only for those diseases where both indicators should be
advisable;
- an inefficient use of the available sources of collected information and allocated resources.
Lastly, it should not be forgotten that the legal framework for accessing and processing the available data from many different sources poses obstacles that need to be addressed and solved. The proposed revision of the statistical law (14) will allow Eurostat and the partner countries within the ESS to use both their technical IT capabilities and the legal mandate for working towards this goal.
The pilot studies in 16 Member States (MS) that were analysed for this report have shown the feasibility of the proposed methodology for many of the 105 indicators (both for incidence and prevalence) included in the Eurostat Morbidity Short List (15).
Demands for diagnoses-specific morbidity in EU programmes on public health
The responsibility for the organisation and delivery of health services and healthcare is largely held by the Member States at national and sub-national level. However, the Commission is asked for actions whenever there is a need to complement Member States’ health policies, in particular in areas such as health promotion, prevention, research or dissemination of information by public health data collections within the ESS (European Statistical System).
The provision of statistics on public health is closely linked to the Community Action Programme in the field of public health 2008–2013, which covers health status including morbidity and is implementing the strategy ‘Together for health: a strategic approach for the EU 2008-2013’ (COM (2007) 630) (16). The most relevant point addressing the importance of health data is the following: ‘… Finally, health policy must be based on the best scientific evidence derived from sound data and information, and relevant research. The Commission is in a unique position to assemble comparable data from the Member States and regions and must answer calls for better information and more transparent policymaking, including through a system of indicators covering all levels (national and sub-national)’ (page 4).
In an ageing society diagnosis-specific morbidity statistics are of particular importance in view of addressing issues such as self-management of multi-morbidity and prevention of long-term care. Time-trends from morbidity statistics will be a major pillar to enhance information and knowledge as requested by the proposed Regulation for establishing a ‘Health for Growth’ Programme, the third multi-annual programme of EU action in the field of health for the period 2014–20 (17). In fact, in the objectives it is stated that ‘… the Programme will support actions on Health information and knowledge to contribute to evidence-based decision making, including collecting and analysing health data and wide-ranging dissemination of the results of the Programme’ (page 7).
The challenge of increasing costs of health in EU economic programme
A population in bad health status is likely to cause higher overall expenditure due to both direct and indirect costs linked to ill-health, such as more people partially or totally inactive during their productive working years, as well as a burden from unhealthy retired people.
Spending on health is not just a cost; it is an investment in order to reduce the burden from diagnosis- and treatment-related costs related to diseases and their derived limitations, impairments and disabilities. Some examples (although not exhaustive) are chronic diseases (diabetes, mental disorders, neurodegenerative conditions, coronary heart disease, cancers, etc...) or diseases impairing the productive years of younger people, such as injuries and their long-lasting consequences.
The recent report on ageing from ECFIN clearly highlights the lack of comparable, quantifiable measures of health status (morbidity) required to evaluate the most likely possible scenario for estimating projections of health care costs in the EU. Providing that data on health expenditure are available, it is assumed that age/gender specific expenditure profiles provides a proxy for health status (i.e. morbidity). In other words, higher expenditure captures higher morbidity (18).
The ageing process in the EU is likely to raise demand for healthcare while also decreasing the working population. This could result in an increase in healthcare spending of 1 % to 2 % of GDP in Member States by 2050. On average this would amount to approximately a 25 % increase in healthcare spending as a share of GDP based on the present health expenditure which ranged from 6 to 12 per cent of GDP in 2009. However, Commission projections show that if people can remain healthy as they live longer, the rise in healthcare spending due to ageing would be halved.
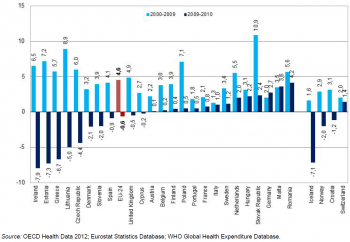
On average across EU MS, health spending per capita increased by 4.6 % per year in real terms between 2000 and 2009, but this was followed by a reduction of 0.6 % in 2010, consequent to the current economic crisis
(15) https://circabc.europa.eu/sd/d/cad2be69-b24e-4e60-84fc-ed5d9370d027/Diagnosis-specific-morbidity-(European shortlist 6 March 2007).xls (16) http://ec.europa.eu/health/strategy/white_paper/index_en.htm (17) http://ec.europa.eu/health/programme/docs/prop_prog2014_en.pdf (18) The 2012 Ageing Report: Underlying Assumptions and Projection Methodologies European Economy 4|2011. (DG ECFIN) http://ec.europa.eu/economy_finance/publications/european_economy/2011/pdf/ee-2011-4_en.pdf.
Acknowledgments
Production
Authors and members of the Task Force ‘Morbidity’: Monica Pace (1)(2), Hartmut Buchow (1), Margarida Domingues de Carvalho (1), Willem Aelvoet (2), Jacques Bonte (2), Gráinne Cosgrove (2), Rita Gaidelyte (2), Mika Gissler (2), Georgeta-Marinela Istrate (2), Merike Rätsep (2), Ieva Strele (2), Bogdan Wojtyniak (2).
Former members of the Task Force ‘Morbidity’: Prof. Howard Meltzer (3), Anne Fagot-Campagna (3), Jean-Marc Schaeffer (1).
Layout and dissemination
Isabelle Fiasse (4).
(1) Eurostat, Directorate F ‘Social Statistics’.
(2) Monica Pace is Seconded National Expert from the Italian National Institute of Statistics, Willem Aelvoet is from the Belgian Federal Public Service Health, Jacques Bonte Private Expert, Gráinne Cosgrove is from the Irish Department of Health, Rita Gaidelyte is from the Lithuanian Institute of Hygiene, Mika GIissler is from the Finnish National Institute for Health and Welfare, Georgeta-Marinela Istrate is from the Romania National Institute for Statistics, Merike Rätsep is from the Estonian National Institute for Health Development, Ieva Strele is from Riga Stradins University, Riga, Latvia, Bogdan Wojtyniak is from the National Institute of Public Health-National Institute of Hygiene, Warsaw, Poland.
(3) Former members of the Task Force Morbidity: Prof. Howard Meltzer was from the Department of Health Sciences College of Medicine, Biological Sciences and Psychology University of Leicester, UK (member until September 2012), Anne Fagot-Campagna was from the French Institute for Health Surveillance (member until March 2012), Jean-Marc Schaeffer Eurostat, Directorate F ‘Social Statistics’ (member until April 2012).
(4) Eurostat, Directorate B ‘Methodology; corporate statistical and IT services’.
See also
Further Eurostat information
Data visualisation
- Regional Statistics Illustrated - select statistical domain 'xxx' (= Agriculture, Economy, Education, Health, Information society, Labour market, Population, Science and technology, Tourism or Transport) (top right)
Publications
Publications in Statistics Explained (either online publications or Statistics in focus) should be in 'See also' above
Main tables
- Title(s) of second level folder (if any)
- Title(s) of third level folder (if any)
Database
- Title(s) of second level folder (if any)
- Title(s) of third level folder (if any)
Dedicated section
Methodology / Metadata
<link to ESMS file, methodological publications, survey manuals, etc.>
- Name of the destination ESMS metadata file (ESMS metadata file - ESMS code, e.g. bop_fats_esms)
- Title of the publication
Source data for tables, figures and maps (MS Excel)
Other information
<Regulations and other legal texts, communications from the Commission, administrative notes, Policy documents, …>
- Regulation 1737/2005 (generating url [http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32005R1737:EN:NOT Regulation 1737/2005]) of DD Month YYYY on ...
- Directive 2003/86/EC (generating url [http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003L0086:EN:NOT Directive 2003/86/EC]) of DD Month YYYY on ...
- Commission Decision 2003/86/EC (generating url [http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003D0086:EN:NOT Commission Decision 2003/86/EC]) of DD Month YYYY on ...
<For other documents such as Commission Proposals or Reports, see EUR-Lex search by natural number>
<For linking to database table, otherwise remove: {{{title}}} ({{{code}}})>
External links
Notes
- ↑ The definition of European statistics is according to Article 2 (2) of the Commission Decision 2012/504/EU of 17 September 2012 on Eurostat.
- ↑ Chan M, Kazatchkine M, Lob-Levyt J, et al. Meeting the demands for results and accountability: a call for action on health data from eight health agencies. PLoS Med 2010; 7: e100023.
[[Category:<Health>|Morbidity statistics methodology pilot studies - introduction]] [[Category:<Statistical article>|Morbidity statistics methodology pilot studies - introduction]]