Calling all data holders: register your data source to help unlock the power of real-world data

date: 17/06/2024
The HMA-EMA Catalogues of RWD sources and studies are two metadata repositories that describe RWD sources and studies which utilise such data to generate real-world evidence. These catalogues enable researchers, pharmaceutical companies and regulators to identify and utilise RWD to investigate the use, safety and effectiveness of medicines.
Our goal is to make the Catalogue of RWD sources a central repository containing metadata information from data sources across Europe and beyond. With a particular focus on use for medicines regulatory purposes, the Catalogue of RWD sources serves as a primary resource for identifying suitable data sources for non-interventional studies.
By registering data sources, we aim to:
· Increase the possibility of identifying relevant data sources as suitable sources for non-interventional studies, and promote collaborations and utilisation of the most appropriate data source(s) in such studies;
· Enhance the visibility of available data sources, making them known to regulators, researchers, and pharmaceutical companies;
· Allow regulators to better interpret study results based on specific data sources as part of a regulatory procedure;
· Link them to studies registered in the Catalogue of RWD studies which have utilised these data sources and therefore promote transparency in observational research;
· Complete the first step required in the expression of interest to become a data partner for DARWIN EU.
Data holders can add information about their data source directly to the Catalogues or send an email at RWDcatalogues@ema.europa.eu.
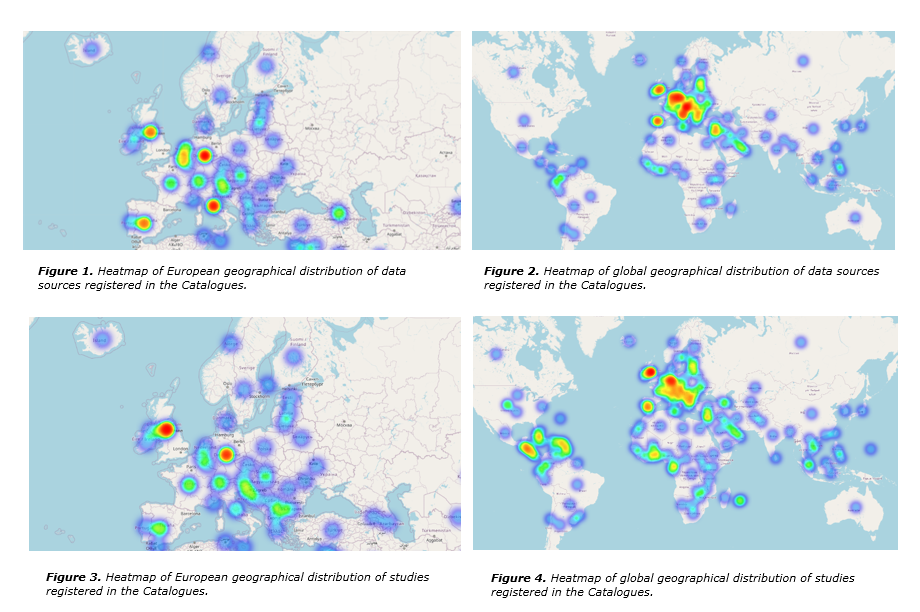
Distribution of data sources and studies registered in the Catalogues
As of June 2024, the RWD Catalogues contain 216 registered data sources and 2840 studies geographically distributed across various regions of the world (Figure 2, Figure 4). A data source or study may contain data from more than one country.
Europe has the highest number of data source and study registrations, with Germany (58 data sources), Italy (57), the United Kingdom and Spain (53) as the top countries leading in data sources (Figure 1). The United Kingdom (987 studies), Germany (854) and Spain (741) are the top three for study registrations (Figure 3). Outside of Europe, the most represented countries in data sources are the United States (14), Israel (12) and Australia (11) (Figure 2) and in studies - the United States (692), Canada (201) and Japan (128) (Figure 4).
Have you already used the RWD Catalogues?
We value your feedback and insights on future improvements that could enhance the Catalogues' usability. Please share your ideas by emailing us at RWDcatalogues@ema.europa.eu
To learn more about the Catalogues and how to use them, you can access the following resources:
· Multi-stakeholder webinar (March 2024)
· Good Practice Guide for the use of the Metadata Catalogue of Real-World Data Sources (public consultation version)