Effects of Biocides on antibiotic resistance
1. What are biocides and how widely are they used?
- 1.1 How are biocides defined?
- 1.2 When are bacteria considered resistant?
- 1.3 How do biocides act?
- 1.4 How widely are biocides used in Europe?
1.1 How are biocides defined?
Bacteria can be killed or inhibited by different antimicrobial products, namely antibiotics that act against infections in humans or animals and biocides such as disinfectants, antiseptics and preservatives.
According to the Biocides
Directive (98/8/EC), biocidal products are intended to destroy,
render harmless, prevent the action of, or otherwise exert a
controlling effect on any harmful organism by chemical or
biological means. The 23 product types covered by the directive
range from drinking water
disinfectants, through wood
preservatives and
insecticides, to
antifouling products (see
full table![]()
Table: 23 Biocidal products listed in Annex V of the Biocides Directive (98/8/EC)![]()
Only biocidal products that act against bacteria are the focus of this assessment and not biocides used to control other micro-organisms such as fungi, protozoans, plants or other animals. More...
1.2 When are bacteria considered resistant?
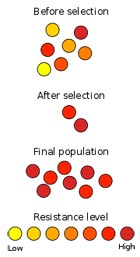
Resistant bacteria can survive biocide concentrations that would kill others.
Although antimicrobial products are used in concentrations that are usually sufficient to inhibit or kill the bacteria treated, some strains of bacteria are able to survive and even grow at these concentrations; they are said to be “resistant”.
Bacteria are considered resistant to antibiotics or biocides in any of the following situations:
- when a strain is not killed or inhibited by the antimicrobial concentration typically used in practice,
- when a strain is not killed or inhibited by a concentration at which the majority of strains of that micro-organism are affected
- when bacterial cells are not killed or inhibited by a concentration acting upon the majority of cells in that culture.
In some cases, resistance mechanisms against biocides can contribute to resistance to antibiotics.
Bacteria are called “insusceptible” when they have natural (innate) properties, such as a specific envelope structure, that impairs biocide penetration. Bacteria develop “tolerance” if they become less affected by a biocide concentration that is active on susceptible strains, so that higher concentrations of the biocide are needed to stop them multiplying.
Bacteria can transfer diverse bits of genetic material (plasmids, transposons, etc.) to other bacteria containing several associated genes. When genetic information coding for different antimicrobial resistance mechanisms is transferred to a new host it is referred to as “co-resistance”.
“Cross-resistant” bacteria are those that have developed survival methods that are effective against different types of antimicrobial molecules having the same mechanism(s) of action.
The term Multi-Drug Resistance (MDR) is used when a bacterial strain is resistant to several different antimicrobial classes. More...
1.3 How do biocides act?
There are many biocidal substances in the market that act in different ways and sometimes several biocides are combined in a product to increase the overall effectiveness. Ideally, the combined action of all the biocides in a product should be greater than the sum of the individual actions (synergy). Biocidal products contain many different molecules and they can all affect how well the product works.
Moreover, some of the components that are added to many household products for a variety of purposes – such as surfactants or membrane permeabilisers - may increase the efficacy of biocides in killing bacteria.
This assessment focuses on the most commonly used biocides for which information on bacterial resistance is available. More...
Table 2: List of active substances in biocidal products and their mode of action![]()
1.4 How widely are biocides used in Europe?
The use of antibiotics in human and animal health care is monitored regularly but the same is not true for biocide use.
Although most biocides are used in large quantities and the volumes produced are many orders of magnitude higher than those of antibiotics, there is no reliable information on the total amounts used in Europe.
The estimated EU market value of biocidal products was €10-11 billion in 2006, and market expansion is expected to continue.
In Europe, biocidal products need to be approved before they are released on the market. Their active ingredients must be safe for humans, animals and the environment. However, even if the products themselves are safe, the fact that they are used in huge volumes could have safety implications. If biocides kill all the bacteria that are reasonably easy to eradicate, the only bacteria left are resistant strains and these are free to grow with no competition from other bacterial populations. It is conceivable that the huge amount of biocides released into the environment alone may already pose a biological threat by applying a selective pressure on bacterial populations, leading to the selection and dissemination of resistant bacteria. More...
The Three-Level Structure used to communicate this SCENIHR Opinion is copyrighted by GreenFacts asbl/vzw.