Filter by
JRC news and updates (1779)
RSS
The report provides examples of how the JRC has made a positive impact on policymaking and society in the past year.

The report analyses 10 key areas ranging from geopolitics, the environment and economics, to technology and social solidarity.
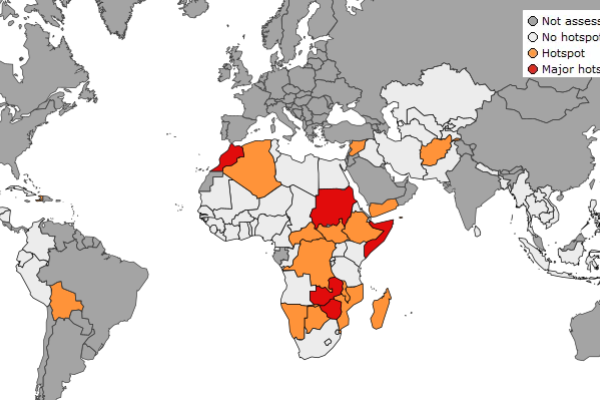
The March edition of the JRC's Anomaly Hotspots of Agricultural Production (ASAP) assessment is now available.

Every euro invested during the 2014-2027 funding programmes will generate additional GDP in the EU.
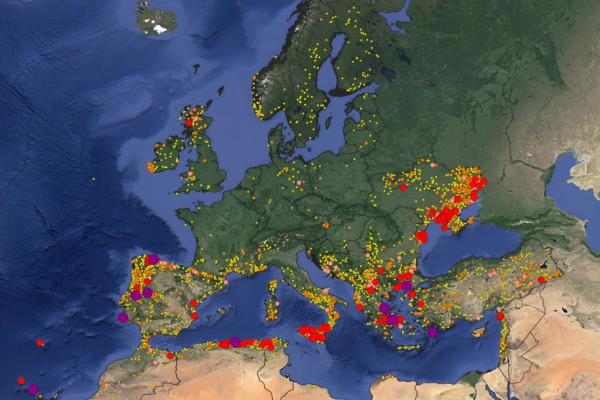
There was a sharp increase in burnt areas in the summer months, mostly affecting the Mediterranean.

According to the March edition of the JRC MARS Bulletin crop monitoring in Europe, winter crop areas in several parts of Europe have been negatively impacted by unfavourable weather conditions since the start of the season. In the north, the most severely affected fields are expected to be resown wi

New reference method for safe exposure.

Novel, underwater detectors are being installed to monitor radon spikes in earthquake-prone locations across Europe. Checking the data against seismic activities could provide clues for predicting earthquakes.

A deep dive into workers’ participation in digital skills training? Findings and implications for policy.

The data hub of the EU Digital Finance Platform is a new at tool for smooth data exchange among national supervisors and financial firms. JRC analysis confirms the synthetic data used to build the database is fit for purpose.
