4. Does development of nicotine addiction depend on the dose?
- 4.1 What happens to nicotine?
- 4.2 How does nicotine exert its effects?
- 4.3 What is the evidence on addictiveness of nicotine?
4.1 What happens to nicotine?
The SCENIHR opinion states:
Nicotine is the principal component alkaloid of tobacco, occurring throughout the plant (Nicotiana tabacum), especially in the leaves. The plant and the compound are named after Jean Nicot, a French ambassador to Portugal, who sent tobacco seeds to Paris in 1550. Crude nicotine was known by 1571, and the compound was obtained in purified form in 1828; the correct molecular formula was established in 1843, and the first laboratory synthesis was reported in 1904. It is one of the few liquid alkaloids; colourless and extremely toxic. Nicotine is commercially obtained from tobacco scraps; it has been used as an insecticide and as a veterinary vermifuge.
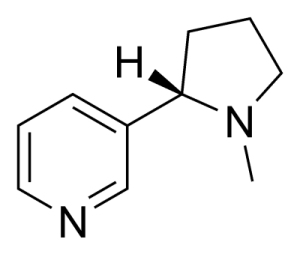
Figure 1 Structure of nicotine (CAS number 54-11-5)
General pharmacodynamic (physiological) effects
Nicotine administration induces a series of multifaceted effects which show great interindividual variability, i.e. the effects vary greatly from person to person. This is reflected in a non-linear and complex dose-response relationship ensuing from a summation of stimulatory and inhibitory actions in the central and peripheral nervous systems.
Low doses of nicotine, including those in the range of inhaled cigarette smoke (1-2 mg), produce stimulation of ganglionic neurotransmission (vegetative ganglia). This generates a complex response which results from a mix of sympathetic and parasympathetic actions. Thus, tachycardia and rise of blood pressure are to a large extent the consequence of sympathetic ganglia activation that induces an increased adrenaline release in the adrenal medulla (via splanchnic nerve stimulation). At the same time, the nicotine action on the carotid and aortic chemoreceptors and on the brain regulating centres modifies the cardiovascular effects determining the great variability observed in the final response. Therefore, the direct nicotine effects on heart rate and blood pressure are rapidly counterbalanced by the peripheral and central cardiovascular compensatory reflexes. Similarly, nicotine–induced activation of parasympathetic ganglia and cholinergic terminals causes an increase of the gastrointestinal peristalsis. In susceptible subjects, first doses may cause nausea, vomiting and related effects of hypercholinergic activation. Nicotine also increases blood glucose levels and the activity of exocrine glands. In the brain, nicotine is clearly a stimulant at low doses. It produces a pattern of alertness in the electroencephalogram (EEG), mediates fast synaptic transmission, and positively modulates a range of cognitive functions. As a result, it improves attention, learning, arousal, motor skill, facilitates memory functions and decreases irritability and anxiety, among other central nervous system (CNS) functions (Balfour and Fagerström 1996, Benowitz 2008, Fattinger et al. 1997, Grybko et al. 2010). An important pharmacological characteristic of nicotine is the rapid development of tolerance to its unwanted effects. Although there is a great individual variability, in many cases tolerance to the peripheral effects appears a few days after the first exposure (Benowitz 2008).
Toxicity effects
At high doses, after the initial stimulation, nicotine rapidly produces a ganglionic blockade due to the inhibition of transmission, which is a consequence of a persistent depolarisation of all autonomic ganglia. This depression of all autonomic ganglia results in bradycardia, hypotension, impairment of adrenaline release, etc. Similarly, a biphasic nicotine-induced action is also observed in the adrenal medulla (a discharge of catecholamines is evoked by small doses whilst their release is blocked by larger doses). It should be noted that most peripheral effects are influenced by compensatory reflexes. In the CNS large doses induce a generalised mental depression, tremors, nausea, and convulsions. The acute lethal dose of nicotine in an adult human is estimated to be about 60 mg (Benowitz 2008, García-Estrada and Fischman 1977, Solarino et al. 2010). This dose (less than 1 mg/kg) is derived from old reported cases of intoxication when nicotine was widely used as an insecticide (Grusz-Harday 1967, Lockhart 1939). In rats the LD50 is ~50 mg/kg and in mice ~3 mg/kg (Okamoto et al. 1994). Acute nicotine poisoning has occurred in children who accidentally ingest tobacco or are occupationally exposed to wet tobacco leaves. Children have played a role, and they continue to do so in many places, in agricultural production of tobacco, where absorption of nicotine from the plant is likely to happen. This nicotine-induced acute condition is known as green tobacco sickness. Clinical features are similar to those observed in adults (Gehlbach et al. 1974, McKnight and Spiller 2005). Ingestion of tobacco products is a major reason for infant and child toxic exposures reported to poison control centres. The large majority (90%) of such accidental poisonings in the population involve children up to 6 years of age (Connolly et al. 2010). However, ingestion of cigarettes and cigarette butts by children aged ≤ 6 years resulted in minor toxic effects (CDC 1997).
Malizia et al. (1983) described four children who ingested two cigarettes each and developed salivation, vomiting, diarrhoea, tachypnoea, tachycardia, and hypotension within 30 minutes, and depressed respiration and cardiac arrhythmias within 40 minutes. Convulsions occurred within 60 minutes of ingestion. All recovered after gastric lavage with activated charcoal, intermittent positive pressure ventilation, and 5 mg diazepam intravenously for convulsions.
A prospective review of 51 cases of tobacco ingestion and five cases of nicotine resin chewing gum exposure was conducted to evaluate the incidence and degree of toxicity caused by these products in children. A dose-response relationship was observed for cigarette exposures. Nine of 10 children ingesting more than one cigarette or three cigarette butts developed signs or symptoms (Smolinske et al. 1988).
4.2 How does nicotine exert its effects?
The SCENIHR opinion states:
The nicotinic acetylcholine receptor
Nicotine acts on a class of cholinergic receptors which are ligand-gated ion channels (nicotine acetylcholine receptors: nAChR). These kinds of receptors are structurally similar to the ones operated by GABA, glycine, glutamate, 5-HT3, etc. Nicotine binding to the nAChR opens the channel and increases its ionic permeability for monovalent cations (Na+, K+) and divalent cations (Ca2+, Mg2+), although with difficulty for the latter and depending on the subtype of nAChR. Neuronal nAChR embrace a conjunct of at least 20 homologous subtypes that mediate fast synaptic transmission throughout the central and peripheral nervous systems (Xiu et al. 2009). Neuronal nAChR are pentamers of homomeric or heteromeric combinations of α (α2 to α10) and β (β2 to β4) subunits, which possess different pharmacological and biophysical properties and locations in the brain (Gotti et al. 2006).
The nAChRs in the CNS are localised both in postsynaptic and presynaptic neural membranes. Studies in recent years have shown that the primary site of nicotine action is presynaptic, and that nAChRs facilitate the release of neurotransmitters when localised in non-cholinergic terminals. In fact, nAChRs are present in the terminals of most of the neurotransmitter systems (GABAergic, glycinergic glutamatergic, dopaminergic, serotonergic, etc.). Likewise, nAChRs have been identified, in different densities, in most of the brain areas.
Nine individual subunits of nAChRs in the human brain have been identified and cloned, and they combine in various conformations to form individual receptor subunits. The structure of individual receptors and the subtype composition are not completely understood. Only a finite number of naturally occurring functional nAChR constructs have been identified (Luetje 2004).
The pentameric structure of the neuronal nAChR and the considerable molecular diversity of its subunits offer the possibility of a large number of nAChRs with different physiological properties. The stoichiometry of most nAChRs in the brain is still uncertain (Kuryatov et al. 2000).
For example, the neuronal nAChR subunits on presynaptic terminals of dopamine neurons projecting to the striatum have been fully defined (Luetje 2004), as has the complete subunit composition of four major presynaptic nAChR subtypes in the striatum (Salminen et al. 2004).
It should also be noted that chronic exposure to nicotine induces a marked increase in the density of nAChRs in most neurotransmitter systems and brain areas (Walsh et al. 2008).
Nicotine pharmacokinetics and metabolism
Nicotine as a weak base (pKa = 8.0) is rapidly absorbed across biological membranes where the pH is at physiological (7.4) or slightly alkaline levels. This is the case for nicotine in cigarette smoke when it reaches the lung alveoli (Pankow et al. 2003). The average nicotine content of a cigarette (6-10 mg) delivers about 1 mg of nicotine (0.5-2 mg) systemically through the smoker’s lungs (Henningfield et al. 1993). The pulmonary bioavailability (the amount absorbed from smoke) of inhaled nicotine is 80-90%. After inhalation it reaches high levels in the brain within 10-20 seconds, thus being equivalent to, or even faster than, an intravenous administration (Gourlay and Benowitz 1997, Hukkanen et al. 2005). In both cases the hepatic first-pass effect (metabolism) is avoided allowing higher levels of unmetabolised nicotine to be delivered to the brain. In addition, nicotine easily crosses the blood-brain barrier. In contrast, the buccal and gastric bioavailability of nicotine is low (20-40%) due to the acidic environment at which nicotine is protonated and therefore poorly absorbed through local membranes. Better absorption is obtained in the intestinal mucosa because of its alkaline pH. The liver first-pass metabolism contributes to the impairment of the bioavailability to a great extent. The time of nicotine blood maximal concentration for oral administrations is about 60-90 min. Nicotine bioavailability through the skin is high (75-100%).
Nicotine is widely distributed in the body (liver, kidney, lungs, etc.; with adipose tissue showing the lowest affinity). Brain tissue exhibits a high affinity for nicotine. It has been reported that nAChR binding capacity for nicotine is increased in smokers compared to non smokers (Breese et al. 1997, Perry et al. 1999). This reflects the higher density of nAChRs in the brain of smokers (nicotine-induced up-regulation of nAChRs). However, the quantity of nicotine delivered from the tobacco product which reaches the brain is higher in non dependent smokers than in heavy smokers (Rose et al. 2010a).
The blood half-life (t½) of nicotine after cigarette smoking or intravenous administration is about 2 hours (t½ = 100-150 min). The disposition of nicotine shows a multiexponential elimination (Hukkanen et al. 2005). However cotinine, the main metabolite of nicotine, has a t½ ≈ 19 hours. It was found recently that every puff of a cigarette induces a peak of nicotine in the arterial blood (Berridge et al. 2010) with a t½ of 45 seconds, but that these peaks do not occur in the brain (Rose et al. 2010a). This finding rules out that the lack of efficacy of nicotine replacement therapy (NRT) (e.g. gums or patches) is due to a continuous delivery of nicotine. In the liver nicotine is mostly metabolised in the endoplasmic reticulum by the cytochrome P450 (CYP) system, mainly by CYP2A6 and CYP2B6. The major metabolite produced by CYP through nicotine oxidation is cotinine, which is further converted to cotinine glucuronide and other metabolites. It should be noted that CYP oxidative metabolism of nicotine to cotinine and its glucuronide conjugation are inhibited by menthol, a commonly used cigarette additive. The pathway of nicotine to cotinine represents around 70-80% of nicotine biotransformation in humans and, therefore, is commonly used as a quantitative biomarker of nicotine exposure as well as of CYP2A6 metabolic activity, which exhibits an important variation in function in humans (Benowitz 2008, Dempsey et al. 2004, Hukkanen et al. 2005, Hukkanen et al. 2010). Many other minor metabolites of nicotine are produced by CYP, glucuronidation, demethylation and other enzymatic pathways. These metabolites have no nicotinic activity, with the exception of nornicotine which is produced by N-demethylation of nicotine in humans and other mammals (besides being a major tobacco leaf alkaloid). Although nornicotine is a minor metabolite, it has been shown that after repeated nicotine administration it accumulates in the brain at pharmacologically relevant concentrations acting as agonist on nAChRs but with about 10-fold lower potency (Dwoskin et al. 2001, Hukkanen et al. 2005).
Renal excretion is the major route of elimination of nicotine and its metabolites (>90% of a dose). Unchanged nicotine accounts for about 10%, and nicotine glucuronide and nicotine N’-oxide for about 5% each, of the total nicotine-derived amount present in urine. Trans-3’-hydroxycotinine (35-40%) and its glucuronide (~10%) are the principal nicotine metabolites determined in urine, both after a single dose and in smokers; unchanged cotinine (10-15%), cotinine glucuronide (~15%) and cotinine N’-oxide (~4%) represent the rest of the cotinine metabolic pathway excreted. Small amounts of a large array of nicotine metabolites produced in the minor biotransformation pathways are also detected in urine. Nevertheless, the pattern of nicotine metabolites and their amounts are highly variable in humans due to the important polymorphism of CYPs and the other enzymatic pathways involved in the metabolic disposition of xenobiotics (Benowitz et al. 2006, Benowitz 2008, Hukkanen et al. 2005). It has been suggested that this genetic variation in xenobiotic metabolism, especially that of CYP2A6, has a role in smoking behaviour and nicotine dependence (Malaiyandi et al. 2005).
Conclusions on nicotine pharmacology
The main effect of nicotine (besides its action on the cholinergic system) is the presynaptic release in the brain of neurotransmitters such as acetylcholine, noradrenaline, dopamine, serotonin, glutamate, GABA and opioid peptides. This allows the possibility that many compounds may modify the action of nicotine on the presynaptic nicotine receptors, and consequently modify the activity of nicotine in the brain. There is substantial interindividual variability in the action and metabolism of nicotine and many aspects of its pharmacology are still not fully understood. Nicotine metabolism may be modified by compounds inducing or inhibiting the activity of the cytochrome P450 system and other metabolic pathways, thus determining pharmacokinetic changes. While the half-life of nicotine in the arterial blood is short, nicotine levels in the brain remain at high levels for much longer.
Addictive properties of nicotine
Nicotine exposure produces adaptive changes in the central nervous system (CNS) leading to an addictive process characterised by compulsive tobacco use, loss of control over tobacco consumption despite the harmful effects, the appearance of withdrawal symptoms upon the cessation of tobacco smoking, and relapse after periods of abstinence (McLellan et al. 2000). As in other addictive processes, the initiation of nicotine addiction has been related to its capacity to induce rewarding/reinforcing effects. However, the negative consequences of nicotine abstinence have a crucial motivational significance for maintenance and relapse of this addictive behaviour (Koob and Le Moal 2008). The terms “reward” and “reinforcement” are often misused and confused. Reward describes stimuli that have appetitive (desirable) consequences and/or produce a hypothetical pleasurable internal state (hedonia). Reinforcement refers to the ability of a stimulus to promote behavioural responses in order to obtain (positive reinforcement) or to avoid (negative reinforcement) such a stimulus. A drug like nicotine that produces rewarding effects will also promote behavioural responses to obtain the drug, i.e. positive reinforcing effects. On the other hand, the effects induced by a drug can be associated with some particular neutral stimuli. After learning the association, this neutral stimulus becomes a conditioned stimulus associated with the drug that can also promote behavioural responses by itself. Several animal models of drug reward/reinforcement are based on these conditioning processes.
The neurobiology of nicotine addiction is a complex phenomenon in which various transmitter systems are involved (Berrendero et al. 2010). The experimental animal models that have been used to investigate nicotine addiction are mainly models of nicotine reward/reinforcement and have been useful to define the neurobiological substrate involved in this behavioural response that is crucial for the nicotine addictive process. New complex behavioural models that resemble the main diagnosis for drug addiction in humans have been developed more recently (Belin et al. 2008, Deroche-Gamonet et al. 2004, Vanderschuren and Everitt 2004). These models of addiction are extremely complex and have been validated only for cocaine addiction. Due to their complexity, these models have still not been used to investigate the neurobiology of drug addiction. Therefore, all the valuable information currently available about drug addiction, including nicotine addiction, is based on the results obtained in experimental models that evaluate drug rewarding/reinforcing effects (see section 3.9 for details about significance of the models).
Nicotinic acetylcholine receptors subunits and nicotine rewarding/reinforcing effects The mesocorticolimbic system plays a crucial role in the rewarding/reinforcing properties of nicotine (Koob and Le Moal 2008). An important component of this system is the dopamine (DA) projection from the ventral tegmental area (VTA) to the frontal cortex and limbic structures, such as the nucleus accumbens (NAc). Nicotine administration increases DA activity in the NAc and other limbic structures (Di Chiara and Imperato 1988) by direct stimulation of nicotinic acetylcholine receptors subunits (nAChRs) within the VTA (Nisell et al. 1994). α4β2 containing nAChRs located on DA cell bodies contribute decisively to the final activation of VTA DA neurons (Mansvelder and McGehee 2003). Indeed, the administration of selective α4β2 antagonists block nicotine-self-administration in rodents (Grottick et al. 2000). In agreement, mice with the β2 subunit knocked out do not self-administer nicotine (Picciotto et al. 1998). The specific location of nAChRs containing the β2 subunit in the VTA plays a crucial role in the mediation of nicotine reinforcement as demonstrated by genetic studies in mice (Maskos et al. 2005). In addition, α4 knockout mice fail to show nicotine-dependent enhancement of DA release in the NAc (Marubio et al. 2003), whereas a single nucleotide mutation rendering α4 containing nAChRs hypersensitive to nicotine (Tapper et al. 2004) demonstrates that this subunit is sufficient to induce nicotine reward (Tapper et al. 2004). The precise role of the α7 homomeric nAChRs in nicotine reinforcing effects remains unclear since conflicting results have been obtained in mutant mice lacking this subunit and in rodents injected with selective α7 nAChR antagonists (Markou and Paterson 2001, Walters et al. 2006). On the other hand, repeated exposure to nicotine leads to up-regulation and desensitisation of nAChRs (Quick and Lester 2002), which are involved in the development of nicotine tolerance and the appearance of a withdrawal syndrome following smoking cessation. The brain regions underlying nicotine physical dependence have not yet been fully clarified, although an involvement of nAChRs located in the medial habenula and the interpeduncular nucleus has been recently reported (Salas et al. 2009).
Recent genome-wide association studies in humans have revealed a clear linkage between genetic variations in the nAChRs and the risk for nicotine dependence (Bierut 2009). Thus, the region on chromosome 15 that includes the family of α5-α3-β4 nAChR genes has been associated with the development of nicotine dependence (Berrettini et al. 2008, Thorgeirsson et al. 2008) and lung cancer (Amos et al. 2008, Hung et al. 2008, Thorgeirsson et al. 2008). These studies differ on whether the connection between the genetic variant at chromosome 15 and lung cancer is direct (Amos et al. 2008, Hung et al. 2008) or mediated through a modification of smoking behaviour (Thorgeirsson et al. 2008).
Involvement of glutamatergic receptors in nicotine rewarding/reinforcing effects Nicotine stimulates nAChRs on glutamatergic terminals that release glutamate in several brain regions including the VTA (Fu et al. 2000). Glutamate receptors located on postsynaptic DA neurons are critically involved in nicotine reinforcing effects (Liechti and Markou 2008). Thus, nicotine-induced DA release in the NAc is blocked by the administration of NMDA and AMPA ionotropic receptor antagonists (Kosowski et al. 2004). In addition, the blockade of NMDA receptor decreases intravenous nicotine self-administration in rats (Kenny et al. 2009). Several studies have also involved postsynaptic mGlu5 and presynaptic mGlu2/3 metabotropic receptors in nicotine reinforcing effects. Thus, mGlu5 receptor antagonists decrease nicotine self-administration (Paterson et al. 2003) and the incentive motivation for nicotine in rodents (Paterson and Markou 2005). The administration of a mGlu2/3 agonist also decreases nicotine self-administration in rats (Liechti et al. 2007). This last result is in accordance with previous studies showing that presynaptic mGlu2/3 receptors modulate glutamate release in a negative manner (Schoepp et al. 2003). The administration of mGlu5 receptor antagonists (Bespalov et al. 2005) or mGlu2/3 receptor agonists (Liechti et al. 2007) also decreases cue-induced reinstatement of nicotine-seeking in rats. Cholinergic and glutamatergic inputs from the pedunculopontine tegmental nucleus (PPTg) to the VTA seem to play a crucial role in nicotine reinforcement since complete lesion of the PPTg reduces nicotine self-administration (Lança et al. 2000, Picciotto and Corrigall 2002). On the other hand, the negative affective changes of nicotine withdrawal are related to a hyperactivity of corticotropin-releasing-factor neurons in the central nucleus of the amygdala (Bruijnzeel et al. 2007, Panagis et al. 2000) and a decrease of DA activity in the NAc (Hildebrand et al. 1999) that seems to be modulated by the glutamatergic system. Thus, mGlu2/3 receptor antagonists, which increase extracellular glutamate in the NAc, attenuate reward deficits associated with nicotine withdrawal in rodents and could also alleviate the depression-like symptoms related to nicotine abstinence in humans (Kenny et al. 2003, Liechti and Markou 2008).
Involvement of GABA receptors in nicotine rewarding/reinforcing effects DA neurons in the VTA are under the inhibitory control of GABAergic inputs that also participate in nicotine rewarding/reinforcing effects. Hence, the administration of the GABA-B receptor agonists such as baclofen, as well as several GABA-B receptor positive allosteric modulators, decrease nicotine self-administration in rats (Paterson et al. 2004, Paterson et al. 2008). Baclofen also inhibits nicotine-induced conditioned place preference in rats (Le Foll et al. 2008). Although GABA neurons are also activated by nicotine, α4β2 nAChRs located on GABA cells tend to desensitise rapidly during repeated nicotine exposure (Mansvelder et al. 2002). Desensitisation of these receptors following repeated nicotine exposure contributes to the final activation of mesolimbic DA neurons induced by the chronic administration of this drug of abuse. Recent studies have reported that the GABA system also participates in nicotine relapse. Thus, the administration of GABA-B receptor agonists decreases cue-induced reinstatement of nicotine-seeking behaviour in rodents (Fattore et al. 2009, Paterson and Markou 2005). In agreement, baclofen also prevents the reinstatement of nicotine conditioned place-preference triggered by nicotine priming in rats (Fattore et al. 2009).
Endogenous opioid system in nicotine rewarding/reinforcing effects
Nicotine administration has been reported to enhance the release of endogenous opioids in the CNS. Thus, an increased concentration of β-endorphin has been found in the hypothalamus after acute nicotine administration in rodents (Marty et al. 1985). In addition, chronic nicotine administration has been found to increase mRNA expression of prodynorphin and µ-opioid receptors (Wewers et al. 1999) in the striatum (Isola et al. 2008). An enhancement of proenkephalin expression has also been observed in the striatum of mice following acute or chronic nicotine administration (Dhatt et al. 1995).
Nicotine induces opposite responses on anxiety-like behaviour related to the development of nicotine addiction that are modulated by the endogenous opioid system. Thus, nicotine anxiolytic-like effects were blocked by a µ-opioid antagonist, and its anxiogenic-like effects were enhanced by a δ-opioid antagonist (Balerio et al. 2005). In addition, a reduction of nicotine anxiogenic-like effects was reported in knockout mice lacking β-endorphin (Trigo et al. 2009). The opioid system also plays an important role in nicotine rewarding effects. The efficacy of naltrexone on smoking cessation in humans supports the involvement of opioid receptors in nicotine reward (Rukstalis et al. 2005). In rodents, nicotine-induced elevations of extracellular DA levels in the NAc were modulated by the activation of µ-opioid receptors localised in the VTA (Tanda and Di Chiara 1998). In agreement, nicotine rewarding properties were blocked in knockout mice lacking µ opioid receptors (Berrendero et al. 2002) or the proenkephalin gene (Berrendero et al. 2005), revealing an involvement of endogenous enkephalins through the activation of µ-opioid receptors. In addition, proenkephalin knockout mice showed a reduction of nicotine-enhanced DA extracellular levels in the NAc (Berrendero et al. 2005). Mice lacking β-endorphin also showed a reduction of nicotine rewarding effects (Trigo et al. 2009). κ-Opioid receptors and their endogenous ligands modulate nicotine reward in the opposite way to enkephalins and β-endorphins. Hence, knockout mice deficient in the prodynorphin gene showed an enhanced sensitivity to nicotine self-administration, probably due to the modulation of its aversive effects (Galeote et al. 2009). The opioid system is also involved in the development of nicotine tolerance. Thus, chronic nicotine exposure produces cross-tolerance with morphine (Biala and Weglinska 2006, Zarrindast et al. 1999), and increases the functional activity of µ-opioid receptors in the spinal cord (Galeote et al. 2006). In addition, µ-opioid receptor knockout mice developed faster nicotine tolerance than wild-type mice, suggesting that increased activation of µ opioid receptors could be an adaptive mechanism to counteract the establishment of nicotine tolerance (Galeote et al. 2006). The involvement of the opioid system in nicotine withdrawal has also been demonstrated. In humans, the opioid antagonist, naloxone induces somatic signs of withdrawal in heavy chronic smokers (Krishnan-Sarin et al. 1999). In rodents, opioid antagonists precipitate somatic manifestations of withdrawal in nicotine-dependent animals (Balerio et al. 2004). In addition, somatic manifestations of nicotine withdrawal were reduced in mice lacking µ-opioid receptors (Berrendero et al. 2002) or the proenkephalin gene (Berrendero et al. 2005). Different studies also indicate that the opioid system participates in the negative emotional states associated with nicotine withdrawal. Thus, naloxone induced aversive effects in nicotine-dependent rodents, which reflects the motivational manifestations of nicotine withdrawal (Balerio et al. 2004, Watkins et al. 2000).
Involvement of cannabinoid receptors in nicotine rewarding/reinforcing effects Several studies demonstrate that the endocannabinoid system plays an important role in the rewarding/reinforcing effects of nicotine (Maldonado et al. 2006). Indeed, the selective CB1 receptor antagonist rimonabant reduces nicotine self-administration in rats (Cohen et al. 2002) and nicotine-induced conditioned place preference in rats and mice (Le Foll and Goldberg 2004, Merritt et al. 2008). In addition, rimonabant pre-treatment blocks nicotine-enhanced DA extracellular levels in the NAc (Cheer et al. 2007, Cohen et al. 2002) and in the bed nucleus of the stria terminalis (Cheer et al. 2007). Nicotine conditioned place preference was also absent in knockout mice lacking CB1 receptors (Castañé et al. 2002, Merrit et al. 2008). The endocannabinoid system has also been involved in the relapse to nicotine-seeking behaviour (De Vries and Schoffelmeer 2005b). Thus, rimonabant attenuates the reinstatement of nicotine seeking-behaviour induced by nicotine-associated cues (Cohen et al. 2005, De Vries et al. 2005a), and reinstatement of nicotine-induced conditioned place-preference provoked by nicotine priming (Biala et al. 2009). The cannabinoid antagonist AM251 also reduced the reinstatement produced by the combination of nicotine-associated cues and a nicotine priming dose (Shoaib 2008). Based on the behavioural and biochemical results obtained in rodents, several clinical trials were developed to evaluate the efficacy of rimonabant for smoking cessation (STRATUS, studies with rimonabant and tobacco use) (Cahill and Ussher 2007). Rimonabant was effective in obtaining a significant smoking cessation in two clinical trials (STRATUS-NORTH AMERICA and STRATUS-WORLD WIDE), although this effect was not significant in the STRATUS-EUROPE trial. The different clinical trials performed with rimonabant have reported several gastrointestinal and psychiatric side effects including nausea, anxiety and depression. Due to these psychiatric side effects, the European Medicines Agency (EMA) recommended the suspension of the marketing authorisation for rimonabant on 23 October 2008.
Other neurotransmitters involved in nicotine rewarding/reinforcing effects The serotonergic (5-HT) system, mainly through the activation of the 5-HT2c receptor subtype, seems to be involved in nicotine reward/reinforcing by exerting an inhibitory influence on DA activity in the VTA (Di Matteo et al. 1999). Thus, 5-HT2c agonists reduce nicotine-self-administration (Grottick et al. 2001), although responding for food was also attenuated by these antagonists. In contrast, no modification on nicotine-induced conditioned place preference was observed by a 5-HT2c agonist in a recent report (Hayes et al. 2009). On the other hand, tobacco smoke contains monoamine oxidase (MAO) inhibitors which are thought to enhance the reinforcing effects of nicotine. Behavioural studies have confirmed this statement since nicotine self-administration was facilitated in rats pre-treated with MAO inhibitors (Villégier et al. 2006a, Villégier et al. 2007). Recently, the hypothalamic neuropeptides hypocretins acting in the insula have also been involved in nicotine reward (Hollander et al. 2008).
4.3 What is the evidence on addictiveness of nicotine?
The SCENIHR opinion states:
Animal models of nicotine reward/reinforcement have enabled the neurobiological substrate involved in this behavioural response that is crucial for nicotine addictive processes. Similar animal models have been widely used to define the neurobiological substrate of the addictive properties of all drugs of abuse. Results obtained in these models suggest that the neurobiology of nicotine addiction is complex involving various transmitter systems in the CNS. Multiple neurotransmitter pathways are activated by nicotine, including dopaminergic, GABAergic and opioidergic pathways. The complexity of the mechanisms of addiction is further underlined by the involvement of the endocannabinoid system, and the serotonergic system also seems to be involved. Dose-dependency appears to have been shown in animal studies. In general, an inverted U-shaped dose-response has been revealed, which suggests that, such as for other drugs of abuse, the addictiveness of nicotine is not directly linear with the dose. The experimental animal models used for evaluating addiction are described in section 3.9.
Conclusions on nicotine
The action of nicotine on the CNS is multifaceted and the mechanisms of addiction are still poorly understood. There are substantial inter-individual differences in the action of nicotine and in its metabolism, which are in part genetically determined. A number of different compounds may in principle interfere with the binding of nicotine with its receptors, while others may interfere with the metabolism of nicotine via the cytochrome P450 system or other pathways. Addiction to nicotine is difficult to measure directly and is usually assessed experimentally with reference to reinforcement assessed in self-administration paradigms.
Cogeneris SPRL ist Inhaber des Urheberrechts der leserfreundlichen Drei-Stufen Struktur in welcher dieses SCENIHR Gutachten präsentiert ist.

