Medicine use statistics
Data extracted May 2022. The data presented in this article refer to 2019.
Planned article update: July 2027.
Highlights
In each of the EU Member States, women were more likely than men to have used prescribed medicines in 2019.
The proportion of the EU population using prescribed medicines in 2019 was lowest in the age group 15–24 years and highest among those aged 75 years and over.

(%)
Source: Eurostat (hlth_ehis_md1e)
This article presents an overview of European Union (EU) statistics related to the use of prescribed and non-prescribed medicines. All of the data presented in this article come from the European health interview survey which was conducted in 2019. These data are based on surveys of people aged 15 years and over and concern their reporting of the use of medicines during the two weeks prior to the survey.
This article is one of a set of statistical articles concerning healthcare activities in the EU which forms part of an online publication on health statistics.
Full article
Prescribed medicines
Prescribed medicine use higher among women …
Among the EU Member States, the proportion of people having used prescribed medicines during a two-week period ranged from more than 55.0 % in Portugal, Finland, Belgium and Croatia to less than 40.0 % in Italy and Romania; similarly high rates were also observed in Iceland and low rates in Turkey (see Figure 1). Without exception, women were more likely than men to have used prescribed medicines, with this gender difference being narrowest in Cyprus and broadest in Latvia. In part, the difference between men and women can be attributed to the use of contraceptive pills and hormones for menopause.

(%)
Source: Eurostat (hlth_ehis_md1e)
… older people …
In nearly all of the EU Member States, the proportion of the population having used prescribed medicines was lowest in the age group 15–24 years, although the smallest proportion in Ireland was recorded for the age group 25–34 years. In broad terms, the proportion using prescribed medicines increased with age, and peaked in the oldest age group (75 years and over). While the percentage of the population in the youngest age group having used prescribed medicines ranged between 2.1 % in Romania and 34.9 % in Belgium, in the age group 75 years and over it ranged between 69.6 % in Italy and 95.4 % in Czechia.

(%)
Source: Eurostat (hlth_ehis_md1e)
A similar analysis is provided in Table 2, with data for the same age groups presented for men and women separately. For the EU as a whole, in 2019 the shares of people using prescribed medicine were higher for women than for men for all age groups. The apparent gender differences, particularly in younger and middle age groups, can be partly attributed to the use of contraceptive pills and hormones for menopause. The gender difference was particularly strong in the 25–34 years age group where there was a 12.2 percentage points gap. In the other age groups below 55 years – those for people aged 15–24 years, 35–44 years and 45–54 years – the gender difference was 7.4 to 8.89 percentage points (with higher shares for women than men). Among older age groups, the difference narrowed: 4.3 and 3.3 percentage points respectively among those aged 55–64 and 65–74 years. The gender difference was narrowest for men and women aged 75 years and over, at 1.1 percentage points.

(%)
Source: Eurostat (hlth_ehis_md1e)
… and people having completed, at most, lower secondary education
For the EU as a whole, the use of prescribed medicines in 2019 was higher among people having completed, at most, lower secondary education and it was lower among those with a tertiary level of education – see Figure 2.
Most of the EU Member States displayed a similar pattern, although with a number of exceptions, the most notable of which was Estonia where the order was reversed (although the shares were quite similar for all education levels). The other exceptions were where people having completed upper secondary or post-secondary non-tertiary education had the highest or lowest shares of the use of prescribed medicines: this part of the population had the highest share in Czechia, Latvia and Lithuania (as well as in Norway), while it had the lowest share in Greece, France, Italy, Portugal and Finland (as well as in Turkey).

(%)
Source: Eurostat (hlth_ehis_md1e)
When analysing the use of prescribed medicines by level of educational attainment and by sex (see Table 3), nearly all EU Member States reported that the proportion of women using prescribed medicines was lowest among those having completed tertiary education. The only exception was in Finland where the share among women with, at most, a lower secondary education was marginally lower; a similar situation was observed in Turkey. For men, the situation was much more varied, as only in 14 Member States was the lowest share of self-reported use of prescribed medicines observed among men with a tertiary education; in one of these (Malta), this lowest share was the same as for men with an upper secondary or post-secondary non-tertiary education.

(%)
Source: Eurostat (hlth_ehis_md1e)
Non-prescribed medicines
In the EU as a whole, the proportion of people using non-prescribed medicine was lower than the proportion using prescribed medicine. In 2019, this pattern was observed among two thirds of the EU Member States, the exceptions being Czechia, Hungary, Latvia, Slovakia, Estonia, Denmark, Finland, Lithuania and Cyprus. Norway and Iceland also reported a higher proportion of people using non-prescribed medicines.
The share of people having used non-prescribed medicines during the two-week period prior to the 2019 survey ranged from less than 20.0 % in Spain, Italy and Romania to more than 55.0 % in Denmark, Cyprus and Lithuania, with a peak at 70.3 % in Finland. Norway (56.6 %) and Iceland (85.2 %) also reported high shares.

(%)
Source: Eurostat (hlth_ehis_md2e)
An analysis by sex of the use of non-prescribed medicines (Figure 3) shows a similar pattern to that for prescribed medicines, with a higher proportion of women than men making use of these medicines. In 2019, the largest gender differences were observed in the Baltic Member States – Lithuania, Latvia and Estonia –where the gender gap ranged from 18.6 to 20.6 percentage points. The smallest differences were in southern EU Member States, in the range of 3.9 to 5.4 percentage points in Greece, Portugal, Italy and Spain.
The age analysis of non-prescribed medicines shown in Table 4 is very different from that for prescribed medicines shown in Table 1. There may well be different reasons for using prescribed and non-prescribed medicines and possibly different practices among EU Member States in prescribing and reimbursing different groups of medicines. For example, this may concern the use of supplements such as vitamins, minerals or tonics which are not necessarily related to the treatment of diseases and are more often used as non-prescribed medicines. In a majority of Member States (16 of the 27), the lowest proportion of people using non-prescribed medicines was observed in 2019 in the 15–24 years age group. For the EU as a whole, close to one third (30.1 %) of the population aged 15–24 years used non-prescribed medicines. In the Baltic and Nordic Member States, as well as in Hungary and Germany, at least 38.0 % of the population aged 15–24 years used non-prescribed medicines.
Non-prescribed medicine use was highest in 2019 among the EU population aged 35–44 years and 25–34 years, with shares of 35.6 % and 35.0 % respectively. In the oldest age group, comprising persons aged 75 years and over, a somewhat lower share of the EU population used non-prescribed medicines (28.7 %); this was the lowest share among the various age groups presented in Table 4. However, the situation across the EU Member States was varied when analysing the use of non-prescribed medicines among those aged 75 years and over. In Czechia, Estonia, Croatia, Lithuania and Slovakia, this age group had the highest share of use of non-prescribed medicines; this was also the case in Norway and Serbia. By contrast, in Belgium, Germany, Ireland, France, Italy, Luxembourg, Portugal, Slovenia and Sweden, this age group had the lowest share of use of non-prescribed medicines. As such, the age pattern for the EU as a whole and for several Member States was almost the reverse of that observed for the use of prescribed medicines.

(%)
Source: Eurostat (hlth_ehis_md2e)
Non-prescribed medicine use highest among people having completed tertiary education
In direct contrast to the situation with prescribed medicines, the use of non-prescribed medicines in 2019 in most EU Member States was most common among people having completed a tertiary education, and least common among people having completed, at most, lower secondary education – see Figure 4. Cyprus was an exception in that the share of the population using non-prescribed medicines was highest among people with, at most, lower secondary education and lowest among people with an upper secondary or post-secondary non-tertiary education.
In Hungary, the proportion of people having used non-prescribed medicines was 29.1 percentage points higher in 2019 for those having a tertiary education than for those having completed, at most, lower secondary education, by far the largest such difference among the EU Member States. By contrast, Denmark, Romania and Cyprus (the latter had, as noted above, a different pattern of use of non-prescribed medicines from most other Member States) had the narrowest range (less than 2.0 percentage points) for the use of non-prescribed medicines according to educational attainment level.
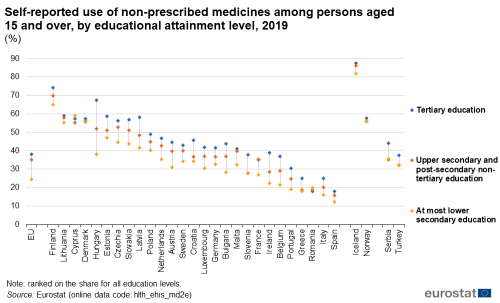
(%)
Source: Eurostat (hlth_ehis_md2e)
Source data for tables and graphs
Data sources
Self-reported data: European health interview survey
The third wave of the European health interview survey (EHIS) is the source of information for the data presented in this article. The general coverage of the EHIS is the population aged 15 years and over living in private households residing in the national territory. This source is documented in more detail in this background article which provides information on the scope of the data, its legal basis, the methodology employed, as well as related concepts and definitions. The interpretation of results between men and women should be done with caution as the data for women include the use of contraceptive pills and hormones for menopause.
The third wave of the EHIS was conducted in all 27 EU Member States, as well as in Iceland, Norway, Serbia and Turkey. The data collection period was generally 2019. However, it was 2018 for Belgium, 2018–2019 for Austria, and 2019–2020 for Germany and Malta.
Context
The European Medicines Agency (EMA) is based in Amsterdam (the Netherlands). Its goals are to facilitate development and access to medicines, evaluate applications for marketing authorisation, monitor the safety of medicines across their life cycle, and provide reliable information about medicines in simple language. All medicinal products for human use have to be authorised by competent authorities before they can be placed on the EU market. Special rules exist for the authorisation of medicines for children, orphan medicines (medicines for rare diseases), herbal products, the ethical use of animals in medicine testing and clinical trials. To ensure that medicinal products are consistently produced and controlled against the quality standards appropriate for their intended use, the EU has set quality standards known as ‘good manufacturing practice’. Once a medicinal product has been authorised for the EU market, its safety is monitored throughout its entire lifespan: this is done through the EU system of pharmacovigilance.
An article based on external trade statistics provides information on international trade in medicinal and pharmaceutical products.
Direct access to
Online publications
Health status
Healthcare activities
Methodology
General health statistics articles
- Health (hlth), see:
- Health care (hlth_care)
- Medicine use (hlth_med)
- European health interview survey (ESMS metadata file – hlth_det_esms)
- European Health Interview Survey (EHIS wave 3) – Methodological manual – 2020 edition
- European Commission – Directorate-General for Health and Food Safety – European core health indicators (ECHI)
- European Commission – Directorate-General for Health and Food Safety, see:
- OECD – Health policies and data
- World Health Organization (WHO) – Global Health Observatory (GHO)
- World Health Organization (WHO) – Health systems governance